在欧洲,诺华与Teva和Generics UK之间的专利纠纷集中在涵盖曲美替尼的片剂剂型的EP2654736B1。2025年4月,上诉委员会因创造性问题撤销了该专利权(T 2424/22)。随后不久,诺华在美国对Teva提起侵权诉讼,涉案专利均为EP2654736B1的同族专利。
2025年6月9日,Teva致函诺华,告知其已向FDA提交 ANDA 申请,申请 FDA 批准其在涉案专利到期之前从事 0.5 mg 和 2 mg 规格曲美替尼片剂的商业化制造、使用和/或销售,Teva还在通知函中声称涉案专利的权利要求均无效和/或不侵权。根据Hatch-Waxman法案,诺华提起侵权诉讼。
诺华请求法院:判定Teva递交ANDA的行为构成侵权、判定FDA批准ANDA的生效日不得早于涉案专利的最晚到期日、在专利到期前发布永久禁令、作出构成侵权的宣告性判决和收取诉讼费用等。
这四项专利均列在橙皮书中,从橙皮书登记信息上看,如果加上儿科用药独占期,其到期日可至2032-07-28。橙皮书中其他专利包括涉及化合物结构式、组合物和适应症专利。
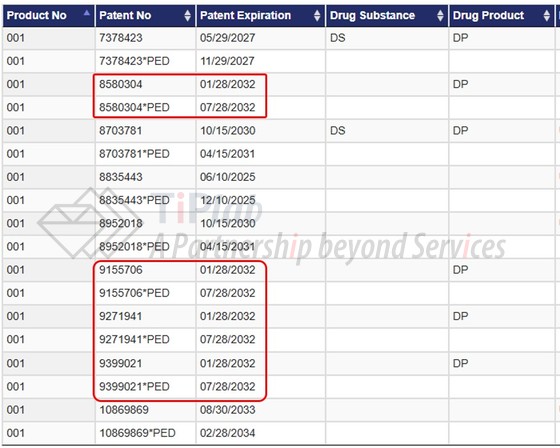
均涉及包含曲美替尼二甲基亚砜溶剂合物的药物片剂,限定了不同的技术特征,包括赋形剂含量、赋形剂基本不含水、药物颗粒的粒径分布、经过微粉化等特征,这些技术特征与EP2654736B1中限定的特征有所区别。之后Teva大概率会提起无效,这些专利在美国是否会被成功无效我们还需静待后续进展。
The ’304 patent claims, inter alia, pharmaceutical tablets comprising trametinib dimethyl sulfoxide solvate, wherein the tablet contains from about 25% to about 89% by weight of one or more excipients, the excipients are substantially free of water, the amount of unsolvated drug does not exceed about 20%, and/or at least 50% of the drug particles have a particle size of 30 micron or less.
The ’706 patent claims, inter alia, pharmaceutical tablets comprising trametinib dimethyl sulfoxide solvate, wherein at least 50% of drug particles have a particle size of 30 microns or less, and/or the drug particles are micronized.
The ’941 patent claims, inter alia, pharmaceutical tablets comprising trametinib dimethyl sulfoxide solvate, wherein the tablets contain from about 25% to about 89% by weight of one or more excipients and the excipients are substantially free of water, and/or at least 50% of the drug particles have a particle size of 30 micron or less.
The ’021 patent claims, inter alia, pharmaceutical tablets comprising trametinib dimethyl sulfoxide solvate, wherein drug particles are micronized, and/or at least 50% of the drug particles have a particle size of 30 micron or less.
* 以上文字仅为促进讨论与交流,不构成法律意见或咨询建议。
